Researchers have developed a new strong, low-cost technique for recycling used cooking oil and agricultural waste to biodiesel, and turning food bits and plastic rubbish into high-value products.
The procedure harnesses a new kind of ultra-efficient catalyst that may create low-carbon biodiesel and other precious complicated molecules out of varied, impure raw materials.
Waste cooking oil currently has to go through an energy-intensive cleaning process to be utilized in biodiesel, since commercial manufacturing methods can only handle pure feedstocks using 1-2% contamination.
The new catalyst is really tough so it could make biodiesel from low-grade ingredients, known as feedstock, containing up to 50% contaminants.
It is so efficient it could double the productivity of manufacturing processes for transforming rubbish like food scraps, microplastics and old tires into high-value chemical precursors used to make anything from medicines and fertilizers to biodegradable packaging.
Co-lead investigator Professor Adam Lee, RMIT, said that conventional catalyst technologies depended on high purity feedstocks and required expensive engineering solutions to compensate for their poor efficiency.
“The quality of modern life is critically dependent on complex molecules to maintain our health and provide nutritious food, clean water and cheap energy,” Lee said.
“These molecules are currently produced through unsustainable chemical processes that pollute the atmosphere, soil and waterways.”
“Our new catalysts can help us get the full value of resources that would ordinarily go to waste – from rancid used cooking oil to rice husks and vegetable peelings – to advance the circular economy.”
“And by radically boosting efficiency, they could help us significantly reduce environmental pollution from chemical manufacturing and bring us closer to the green chemistry revolution.”
Catalyst sponge: advancing green chemistry
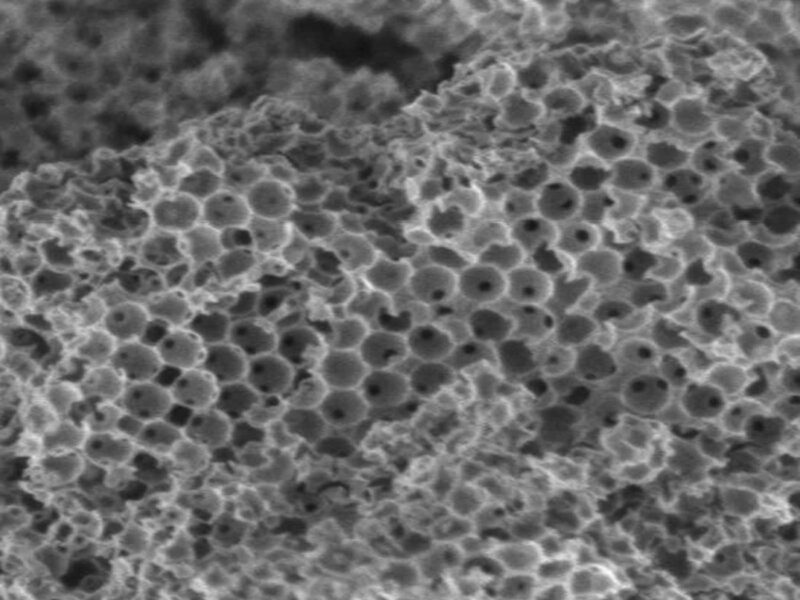
To make the new ultra-efficient catalyst, the group fabricated a micron-sized ceramic sponge (100 times thinner than a human hair) which is highly porous and contains different specialized active elements.
Molecules initially enter the sponge large pores, where they experience a first chemical response, and then move into smaller pores where they undergo another reaction.
That can perform a number of chemical reactions in sequence inside a single catalyst particle, and it might be a game changer for its $34 billion global catalyst market.
Co-lead investigator Professor Karen Wilson, also by RMIT, stated the new catalyst design mimicked the way that enzymes in cells coordinated complex chemical reactions.
“Catalysts have been developed that can perform multiple Simultaneous reactions, however, these approaches offer little control over the chemistry and also are inclined to be inefficient and erratic,” Wilson said.
“This is particularly important in developing countries where diesel is the primary fuel for powering household electricity generators,” Wilson said.
“If we could empower farmers to produce biodiesel directly from agricultural waste like rice bran, cashew nut and castor seed shells, on their own land, this would help address the critical issues of energy poverty and carbon emissions.”
To create a strong and exact method of performing multiple reactions in a set sequence.
“It’s like having a nanoscale production line for chemical responses – all housed in a single, tiny and super-efficient catalyst particle”
DIY diesel: Update distributed biofuel production
The sponge-like catalysts are cheap to manufacture, using no valuable metals.
Making low-carbon biodiesel from agricultural waste together with those catalysts demands little more than a large container, some gentle heating and stirring.
It’s a low-technology, cheap approach that could progress
Is the primary fuel for powering household power generators,” Wilson stated.
“If we could empower farmers to produce biodiesel directly from agricultural waste like rice bran, cashew nut and castor seed shells, on their own land, this would help address the critical issues of energy poverty and carbon emissions.”
Even though the new catalysts can be utilized immediately for biodiesel , with further development they could be easily tailored to produce jet fuel from agricultural and forestry waste, old rubber tires, and even algae.
The next steps to the RMIT School of Science research team are Scaling the catalyst fabrication from grams to kilograms and embracing 3D printing technologies to accelerate commercialization.
“We are also expecting to expand the Assortment of chemical reactions to Include light and electric activation for cutting-edge technologies like artificial photosynthesis and gas cells,” Lee said.
“And we are looking to utilize prospective business partners to Create a range of commercially available catalysts for distinct applications.”
Related Journal Article: https://www.nature.com/articles/s41929-020-00526-5